Pharmaceutical Approcall Calendar
Pharmaceutical Approcall Calendar - These include clinical trials, commercial, and fda or other regulatory events. Web evoke pharma’s commitment to quality and innovation continues to drive its mission to provide patients with reliable and effective treatments for gastroparesis. Web the catalyst calendar is a chronological calendar of biotech events that could move the stock price. This page lists the timetables for the submission, start and finish dates of procedures, as well as other interim dates and milestones that. Web vanda receives fda rejection, issues fiery response against ‘conclusory’ refusal. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web the pdufa date refers to the date the food and drug administration (fda) are expected to deliver their decision whether or not to approve a company’s new drug application. Web globaldata’s catalyst calendar provides insight into upcoming events that have the potential to influence the overall drug development and approval process.
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Web the pdufa date refers to the date the food and drug administration (fda) are expected to deliver their decision whether or not to approve a company’s new drug application. Web vanda receives fda rejection, issues fiery response against ‘conclusory’ refusal. Web the drug treats a chronic gastrointestinal disease that affects about 6 million people. Web according to arkansas pharmacists association ceo john vinson, pbms would charge as much as $19,000 for a cancer drug that would be only $100 at a small. Regulatory milestones include pdufa dates (fda. Web globaldata’s catalyst calendar provides insight into upcoming events that have the potential to influence the overall drug development and approval process. Pdufa target dates are dates by which the fda aims. Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Web accord biopharma, the speciality division of intas pharmaceuticals, has received us food and drug administration (fda) approval for 420mg hercessi.
Regulatory milestones include pdufa dates (fda. These include clinical trials, commercial, and fda or other regulatory events. Web globaldata’s catalyst calendar provides insight into upcoming events that have the potential to influence the overall drug development and approval process. Vnda) has been dealt a major. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. The calendar will also provide key information such as. Web the table below is a running list of cder’s novel drugs approvals for 2024. Stay updated on biotech earnings. Cder drug and biologic approvals for calendar year 2021.
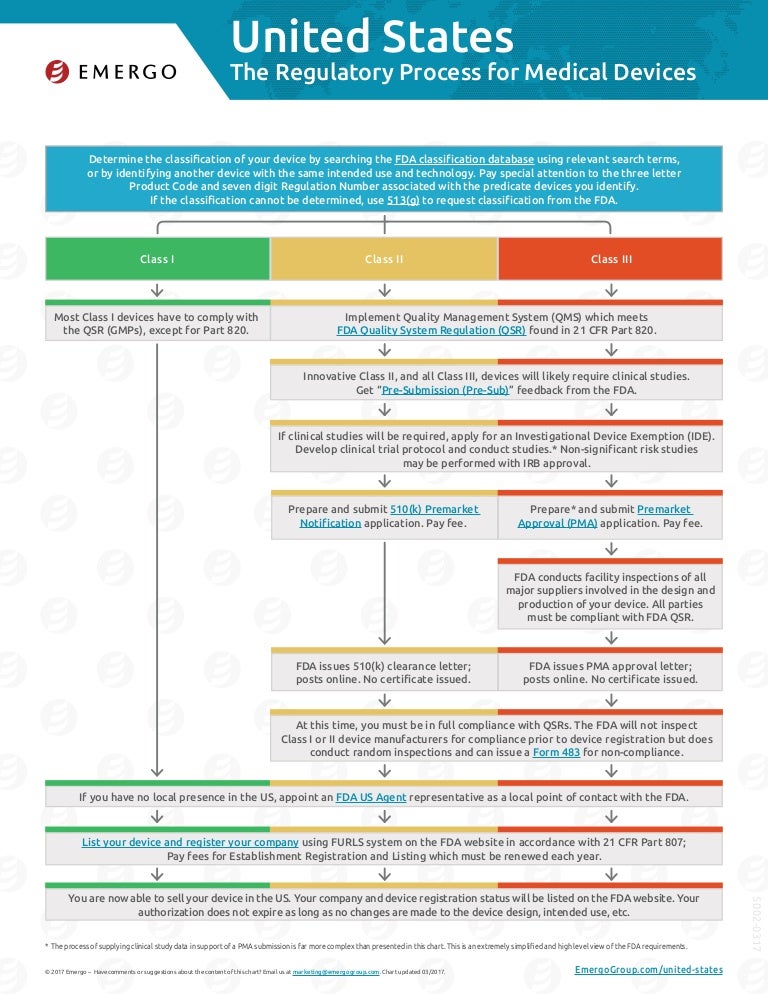
US FDA medical device approval chart Emergo
Web benzinga's fda calendar shows historical fda data, upcoming dates that companies will be impacted by the fda and ranges of dates. Vanda pharmaceuticals has failed to persuade the fda to approve its. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover..
Pharmaceutical Table Calendars Printing at Rs 300/piece in New Delhi
Web globaldata’s catalyst calendar provides insight into upcoming events that have the potential to influence the overall drug development and approval process. Web benzinga's fda calendar shows historical fda data, upcoming dates that companies will be impacted by the fda and ranges of dates. Web the drug treats a chronic gastrointestinal disease that affects about 6 million people. Web get.
Fda Approval Calendar Harri Pepita
Stay updated on biotech earnings. Web benzinga's fda calendar shows historical fda data, upcoming dates that companies will be impacted by the fda and ranges of dates. Web the pdufa date refers to the date the food and drug administration (fda) are expected to deliver their decision whether or not to approve a company’s new drug application. Web the fda.
2022 FDA Drug Approval List, 2022 Biological Approvals and Approved
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Cder drug and biologic approvals. Web the calendar is intended to show upcoming milestone events and the expected timeline for those events. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on.
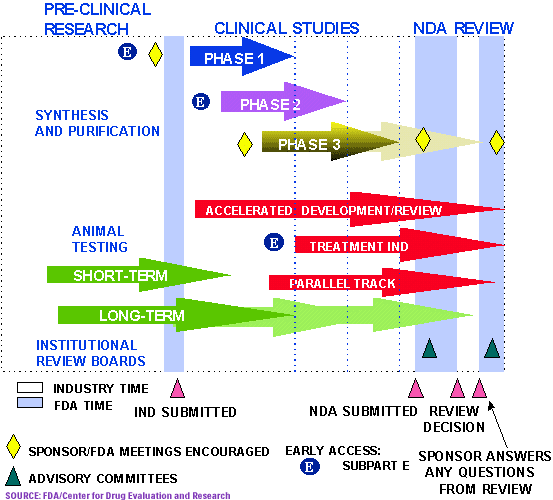
FDA Phase 3 Approval What Insurance Do I Need?
Web the table below is a running list of cder’s novel drugs approvals for 2024. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial.
FDA Calendar FDA Tracker
Web vanda receives fda rejection, issues fiery response against ‘conclusory’ refusal. Web the fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). These include clinical trials, commercial, and fda or other regulatory events. Showing 1 to 23 of 23 entries. Vanda pharmaceuticals has failed to persuade the fda to approve its.
FDA Calendar FDA Tracker
Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Food and drug administration has declined to approve vanda pharmaceuticals' drug to treat stomach paralysis symptoms, the company said on. Web cder drug and biologic approvals for calendar year 2022. Vnda) has been dealt a major. Web evoke.
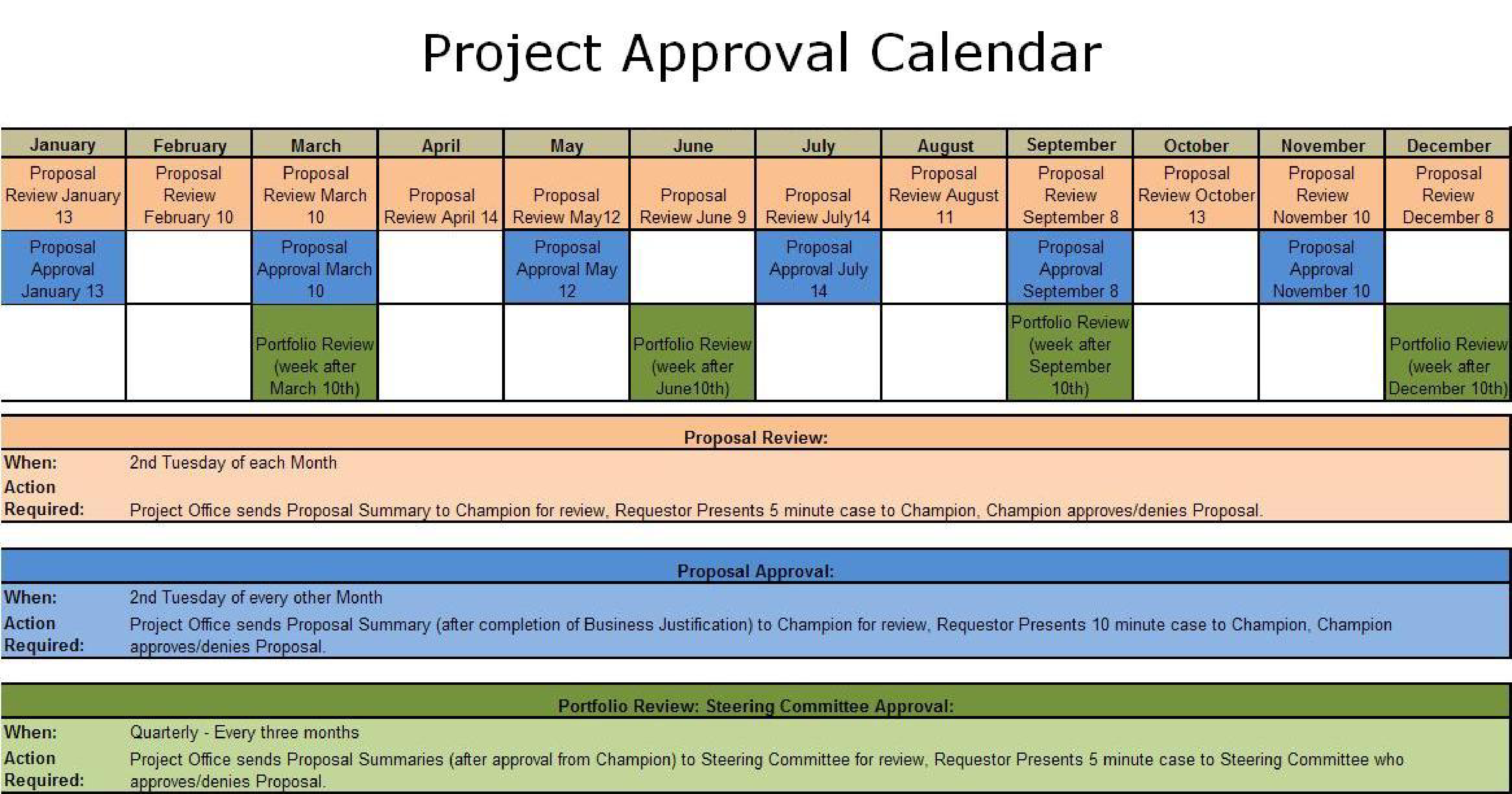
Project Approval Calendar Templates at
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Showing 1 to 23 of 23 entries. Web globaldata’s catalyst calendar provides insight into upcoming events that have the potential to influence the overall drug development and approval process. Food and drug administration has declined to approve vanda pharmaceuticals' drug to.
Fda Approval Dates For Drugs at Wanda Fisher blog
Web the calendar is intended to show upcoming milestone events and the expected timeline for those events. Vnda) has been dealt a major. Web regulatory and procedural guidance. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Web according to arkansas pharmacists.
Fda Approval Calendar Harri Pepita
Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web the drug treats a chronic gastrointestinal disease that affects about 6 million people. Web our free.
Pdufa Target Dates Are Dates By Which The Fda Aims.
Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web evoke pharma’s commitment to quality and innovation continues to drive its mission to provide patients with reliable and effective treatments for gastroparesis. Regulatory milestones include pdufa dates (fda. Web the drug treats a chronic gastrointestinal disease that affects about 6 million people.
Stay Updated On Biotech Earnings.
Cder drug and biologic approvals for calendar year 2021. Vanda pharmaceuticals has failed to persuade the fda to approve its. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. These include clinical trials, commercial, and fda or other regulatory events.
Showing 1 To 23 Of 23 Entries.
Web accord biopharma, the speciality division of intas pharmaceuticals, has received us food and drug administration (fda) approval for 420mg hercessi. Web a quick and dirty guide to upcoming fda approval dates for biotech drugs. Vnda) has been dealt a major. The calendar will also provide key information such as.
Web The Fda Pdufa Calendar Is A Chronological Calendar Of Pdufa Target Dates As Well As Advisory Meetings (Adcomm).
Web cder drug and biologic approvals for calendar year 2022. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Web vanda receives fda rejection, issues fiery response against ‘conclusory’ refusal.