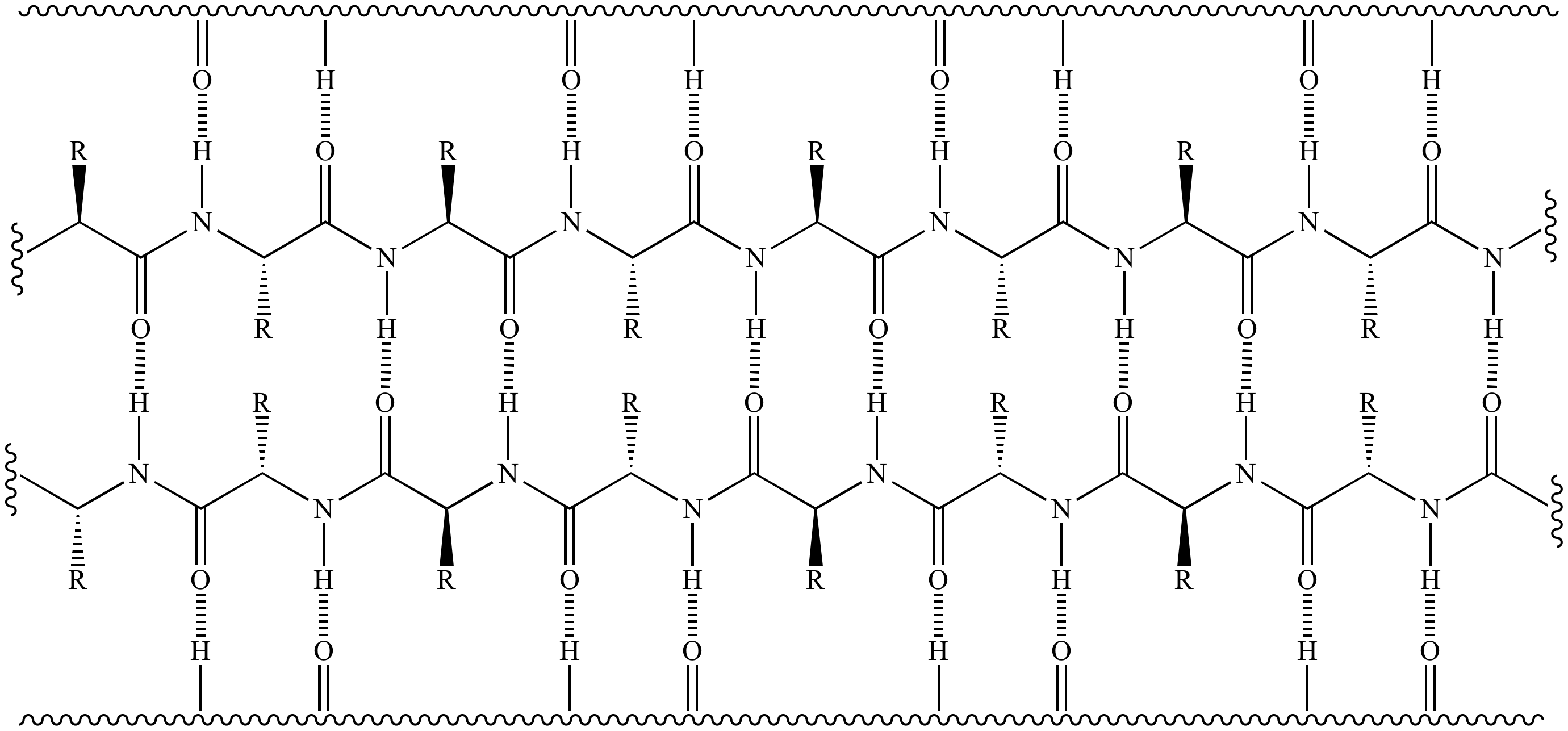
Beta Sheet Hydrogen Bonding
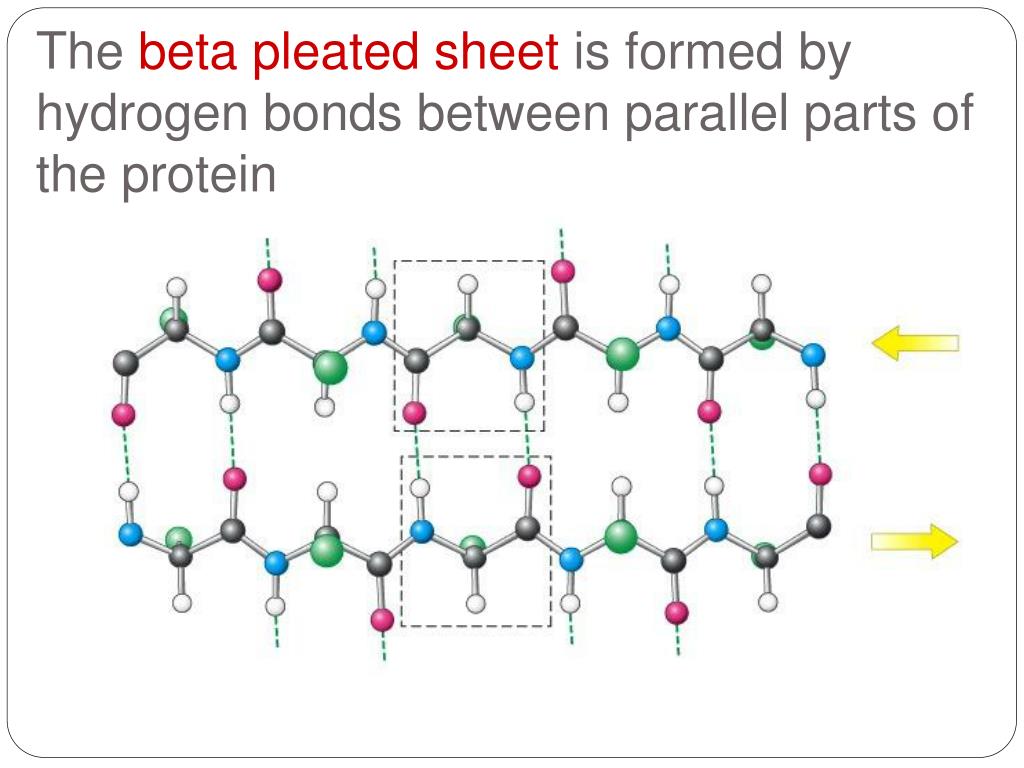
Beta Sheet Hydrogen Bonding - Web the hydrogen bonds are equally distanced. Some other characteristics of ß sheets are displayed below. This structure occurs when two (or more, e.g. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very. The three parallel strands are shown in both cartoon format (left) and in. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. The amino acids are more.
Some other characteristics of ß sheets are displayed below. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very. Web the hydrogen bonds are equally distanced. This structure occurs when two (or more, e.g. The three parallel strands are shown in both cartoon format (left) and in. The amino acids are more. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand.
This structure occurs when two (or more, e.g. The amino acids are more. Some other characteristics of ß sheets are displayed below. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. The three parallel strands are shown in both cartoon format (left) and in. Web the hydrogen bonds are equally distanced. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very.
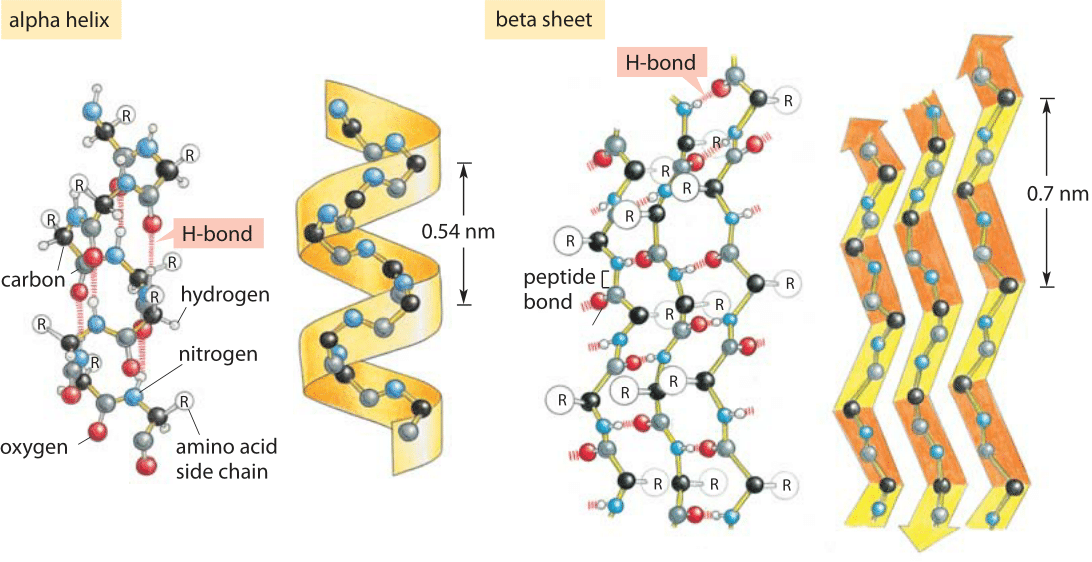
The stable arrangement of hydrogenbonded amino acids in the alpha
This structure occurs when two (or more, e.g. Web the hydrogen bonds are equally distanced. The amino acids are more. The three parallel strands are shown in both cartoon format (left) and in. Some other characteristics of ß sheets are displayed below.
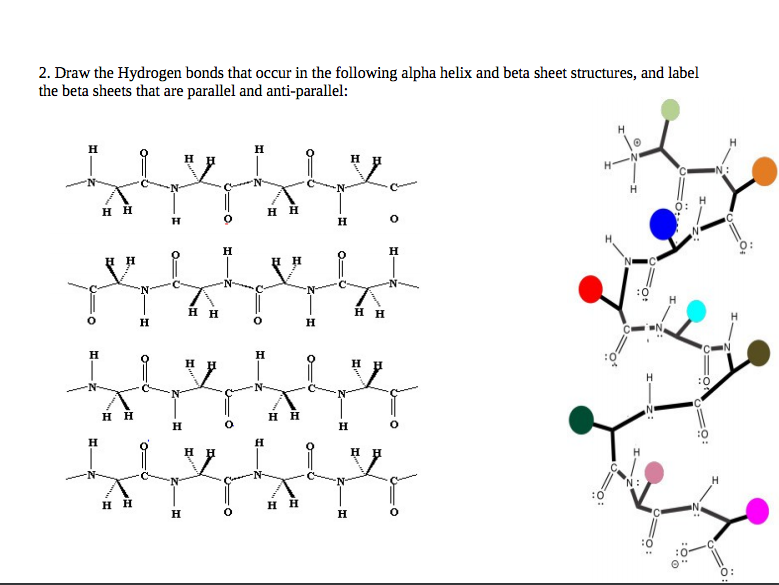
Solved 2. Draw the Hydrogen bonds that occur in the
The amino acids are more. This structure occurs when two (or more, e.g. The three parallel strands are shown in both cartoon format (left) and in. Some other characteristics of ß sheets are displayed below. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein,.
PPT The Protein PowerPoint Presentation, free download ID5591272
Some other characteristics of ß sheets are displayed below. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. The three parallel strands are shown in both cartoon format (left) and in. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and.
Amino Acids 8. The betapleated sheets secondary structure of Proteins
The three parallel strands are shown in both cartoon format (left) and in. Some other characteristics of ß sheets are displayed below. Web the hydrogen bonds are equally distanced. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. The amino acids are more.
Chapter 2 Protein Structure Chemistry
This structure occurs when two (or more, e.g. The three parallel strands are shown in both cartoon format (left) and in. Web the hydrogen bonds are equally distanced. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. Web beta strands (sheets) in which the hydrogen bonds are between.
Hydrogen Bond Analysis Tutorial BioChemCoRe 2018
Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. This structure occurs when two (or.
7.4 Proteins Microbiology 201
Web the hydrogen bonds are equally distanced. This structure occurs when two (or more, e.g. The amino acids are more. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. Some other characteristics of ß sheets are displayed below.
PPT SURVEY OF BIOCHEMISTRY Amino Acids and Proteins PowerPoint
Web the hydrogen bonds are equally distanced. The three parallel strands are shown in both cartoon format (left) and in. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very. Web unlike the α helix, the ß.
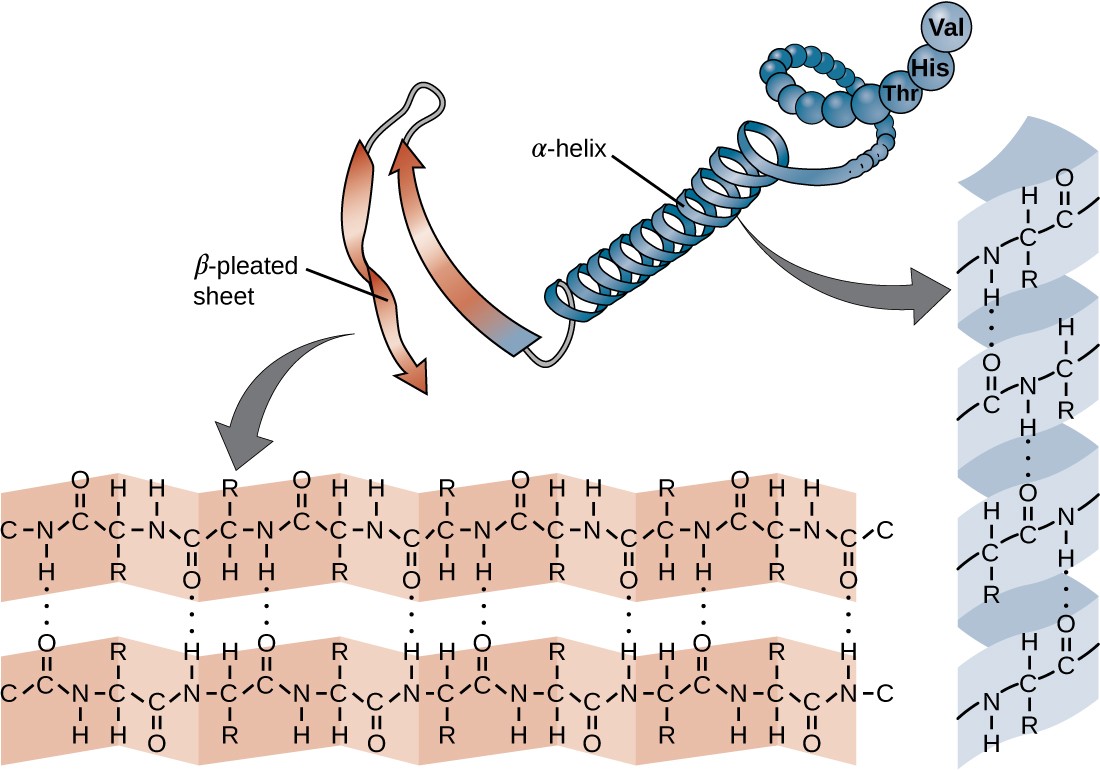
Illustrated Glossary of Organic Chemistry Beta sheet, betapleated sheet
Some other characteristics of ß sheets are displayed below. The three parallel strands are shown in both cartoon format (left) and in. Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very. This structure occurs when two.
Chemical Forums Beta sheet hydrogen bonding
Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. The amino acids are more. The.
This Structure Occurs When Two (Or More, E.g.
Some other characteristics of ß sheets are displayed below. Web the hydrogen bonds are equally distanced. Web unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within a strand. The amino acids are more.
The Three Parallel Strands Are Shown In Both Cartoon Format (Left) And In.
Web beta strands (sheets) in which the hydrogen bonds are between backbone atoms (again amide hs and carbonyl os) on noncontinuous stretches of the protein, and reverse turns, which occur within a very.